Troponin
Troponin and the Thin Filament
The thin filament is composed of three proteins: actin, tropomyosin, and troponin. Actin monomers spontaneously polymerize to form filamentous actin, F-actin, the backbone of the thin filament. F-actin has a double-stranded helical structure and is intimately associated with another filamentous protein, tropomyosin. Tropomyosin is a ~42 nm long molecule that exists as a dimer of two alpha-helical chains stabilized as a coiled coil. Tropomyosin is bound to F-actin and runs the length of the thin filament by overlapping with neighboring molecules in a head-to-tail fashion. The location of tropomyosin on F-actin establishes whether contraction is inhibited or not and is precisely controlled by troponin.
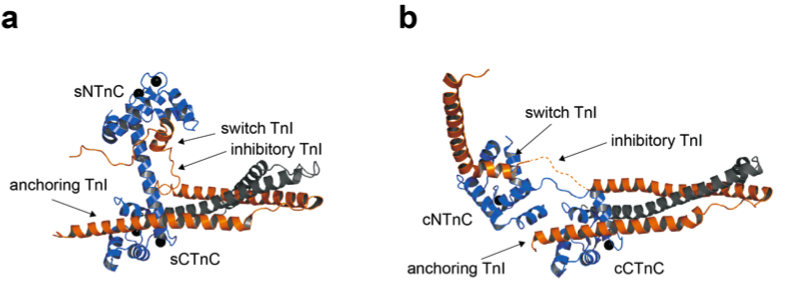
Troponin is a heterotrimeric protein made up of troponin C (TnC), troponin I (TnI), and troponin T (TnT). The exact arrangement of the subunits is dependent on Ca2+ and dictates the myofilament's function. TnC is the calcium binding subunit; it is a bi-lobed protein that has two high-affinity metal binding sites in its C-terminal domain (CTnC) and two low-affinity Ca2+ binding sites in the N-terminal domain (NTnC). A crucial difference between the cardiac and skeletal isoforms of TnC is that in the cardiac isoform, site I of the N-terminal domain is unable to bind Ca2+. TnI is the inhibitory subunit of troponin, and it interacts with TnC, TnT, and actin. The interaction between the N-terminal region, H1 or the 'anchoring region', of TnI and CTnC is thought to be important in tethering the complex to the thin filament and requires that both metal binding sites are occupied with either Mg2+ of Ca2+. The interaction between the 'switch region' (H3) of TnI and NTnC is the regulatory step, and occurs only when Ca2+ is bound to NTnC. When sarcoplasmic Ca2+ levels are low, Ca2+ and the switch region of TnI dissociate from NTnC. The release of TnI from NTnC leads to the interaction of the 'inhibitory' and C-terminal domains of TnI with actin. The interaction between of TnI with actin inhibits contraction by positioning tropomyosin on the thin filament so that the myosin binding sites are blocked. When Ca2+ levels are high, the switch region of TnI drags the inhibitory and C-terminal domains of TnI off actin, tropomyosin moves towards the actin double helical groove, and the actomyosin cross-bridges form. TnT interacts with TnI and tropomyosin, connecting the troponin complex to the thin filament at every seventh actin monomer.